If so, I think you might have missed it.
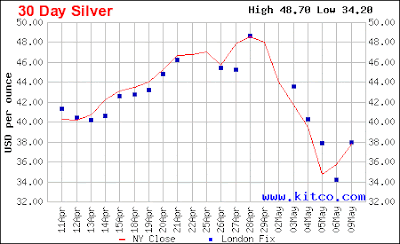
You know all commodities got hammered last week, gold, silver and oil all down, and the dollar up. The minimum appears to have been last week, with silver putting in a 38% retreat before starting back up.
This chart shows silver vs the long term moving average, raw data on the bottom, normalized around that long average on the top. The correction was similar in magnitude to previous corrections, as shown. On the scale of this chart, it looks even more abrupt than it was. This chart, of the last few days, shows it a bit better, stripped of the intra-day highs and lows:
The actual low was apparently late on the 5th or on the 5th/6th transition.
The big question is: is it over? My guess is yes. The severity of the drop is at least partly due to the change in leverage laws that just went into effect, although Denninger says in that link leverage was all that, but the metals markets are famously manipulated by many different players: central banks, big players (cough - JP Morgan), and so on. Manipulation or change in laws, it looks to me like silver had gone up too far, too fast, too soon and needed to re-establish support levels. It has attracted far more speculative froth than gold has lately. Markets don't go up in straight lines for long.
In times like this, I ask myself some fundamental questions: has the big picture changed? Not from what I can see. If it hasn't, and the big picture is still bullish for metals, what's the small picture? Last Thursday, Mrs. Graybeard was trying to get me to say, "go back up the truck and buy" on silver. I wouldn't. Not because I didn't think it was a buying opportunity, but because every analyst had a different idea on where it was going. I figured if it's going to $30, why buy at $36? I don't expect to be able to hit the minimum, but I'll wait until it looks like the drop is slowing, stopping, or even starting back up before I do. I felt comfortable enough by Saturday. When I talk about buying silver, I mean physical silver you can hold in your hands, not paper ETFs or any of that. There's a place for that, too, but physical metal in your possession is king.
I sure don't believe the TV talking heads saying that the precious metal bull market is over. I believe fundamentals would have to change radically before the bull market runs out.
Meanwhile, there's absolutely nothing to change the advice we've all talked about for months now: beans, bullets, band-aids and bullion all will get you through bad times better than none. And I specifically include copper, brass and lead in my investment portfolio. .22LR is the small change of the new millenium!
Oh - and usual disclaimers: I'm an engineer, not an investment advisor. These opinions are worth exactly what you paid for them. Anyone who would take the advice of a random dude on the Internet deserves what they get. Your mileage may vary. Do not remove this tag under penalty of law.


Bought some more Ag on the 5th. Good to know my timing was right this time :-)
ReplyDeleteOn the "bullets" issue, I always figured ammo would make a better barter commodity than gold or silver. If food becomes scarce, folks probably won't trade for gold. Ammo, which can be used to provide food, will be a lot more popular at such times. Back a few years ago, when you could still buy a brick of .22 LR for seven bucks, I bought a goodly number of them. Plenty to use and plenty to barter.
SG,
ReplyDeleteBack on the topic of silver: my mind wanders far and wide (more like stumbling around lost in the forest), and today I found myself considering that less-reputable sellers of silver bullion could produce rounds or bars that were base metal that were then given a heavy plate of real silver, and I would never be able to tell the difference, short of cutting or melting my rounds. Any idea if there is a way a person go about determining if the "mint" was adulterating their coins/rounds/bars or not?
It occurred to me that an engineer who invests may have considered this notion long ago. This is more of an idle thought on my part than a paranoid need to chill any real doubts. I just couldn't think of an obvious solution off-hand.
RegT, density can be used to uniquely identify a metal - weigh it on an accurate balance scale (like a reloading scale - this compares masses not simply measures gravitational force) and then measure or calculate the volume (water displacement would work - and there are several indirect methods as well). Convert the mass into grams or kg and the density in cm^3 (or ml) and divide the mass by the volume. Now you have a density that you can compare to known accepted values to determine if you have pure silver. That would make a pretty simple and non-destructive method of testing at home.
ReplyDelete(there are other properties such as electrical conductance, heat transmission, et al, that could be used to identify a metal - there are reference books that engineers use that are full of physical characteristics of different materials that could be used to identify them if one had the methods to test)
Thanks, LA. I'll try to find a small beaker or other container that is marked more accurately than your standard measuring cup and give that a go, simply for the fun of using a scientific method to answer a question such as this.
ReplyDeleteOn second thought, I have some syringes that are probably fairly accurate in their marking, so if I suck up the displaced water from a container filled to capacity, that should give me a volume. My scales are marked in grains, but I recall that there are 7000 grains in one pound. I'll have to convert for the difference between Troy and Avoirdupois as well, I think. No, just found a site where it lists Troy ounces as weighing 480 grains :-) 1 gr = 0.064799 g, so 1 Troy ounce = 31.1035 grams.
Of course, if I don't like the data I end up with, I can always throw it out, lose it, or bury it in a hockey stick graph . . .
The Chinese were caught making debased gold bars a few years back. Check out the density of Tungsten (W) at 19.25 g/cm^3 vs. Gold (Au) at 19.30 g/cm/^2. Rounded to 3 digits, the densities are the same. Short of drilling through debased gold bars to confirm, it is nearly impossible to detect a bar of gold with a tungsten insert embedded in it. By the quality of the bars, it was thought that the Chinese government was behind these debased gold bars. Not sure if these are still making the rounds between sovereign banks internationally, but it was a problem a few years back. (Some of the gold bars in US Government hands acquired through international circles were found to be debased, with tungsten cores -- they ultimately were traced back to China :-)
ReplyDeleteThere have also been tunsten Krugerands plated with gold available on E-bay at times. They don't look as sharply minted, but could easily fool a non-astute amateur buyer, with the right density to 3 digits. Caveat emptor, the ancient "Eureka" comment not withstanding with regards to using density measurements :-)
- GB
III
It occurred to me that an engineer who invests may have considered this notion long ago. This is more of an idle thought on my part than a paranoid need to chill any real doubts. You can bet that a paranoid engineer would have thought of this!
ReplyDeleteThere was a news story a few months ago, about gold bars that were really tungsten and just 24k plated. I think they came from China. If I remember correctly, density was what made the holder suspicious, and they did some more tests.
A determined, smart counterfeiter could do better, but density is the first thing to measure. It is subject to error, of course, like any measurement.
Most jewelers have something like this gold test kit from Amazon. You abrade some metal onto the little piece of ceramic and drop some solution on it. Silver turns a reddish color, and 90% coins (or sterling) don't turn as vivid red as 99.999 fine silver eagles or other pure silver coins. Plating might abrade off when you do that to expose base metal, especially thin plating.
This is a serious question. Counterfeiting may not be the oldest profession, but it's close.
That's one reason I lean toward old 90% silver coins, usually called junk silver. Someone absolutely could counterfeit them, but it would be a lot of work. They'd have to make molds of different years, in different thicknesses, with different marks, cast them, plate them, finish them, then artificially age them. So I think junk silver coins from a major dealer are most likely safe.
Well, that's not as encouraging as I would have hoped ;-) You make a great case for junk silver. I thought about getting some, but for some reason I can't remember, I decided not to do so. Seduced by those shiny new rounds, I suppose.
ReplyDeleteBTW, I see from your latest post (5/11/11) that it looks as if silver has actually dipped again just a little. I wonder what that is all about?